Constraints and consequences of the emergence of amino acid repeats in eukaryotic proteins
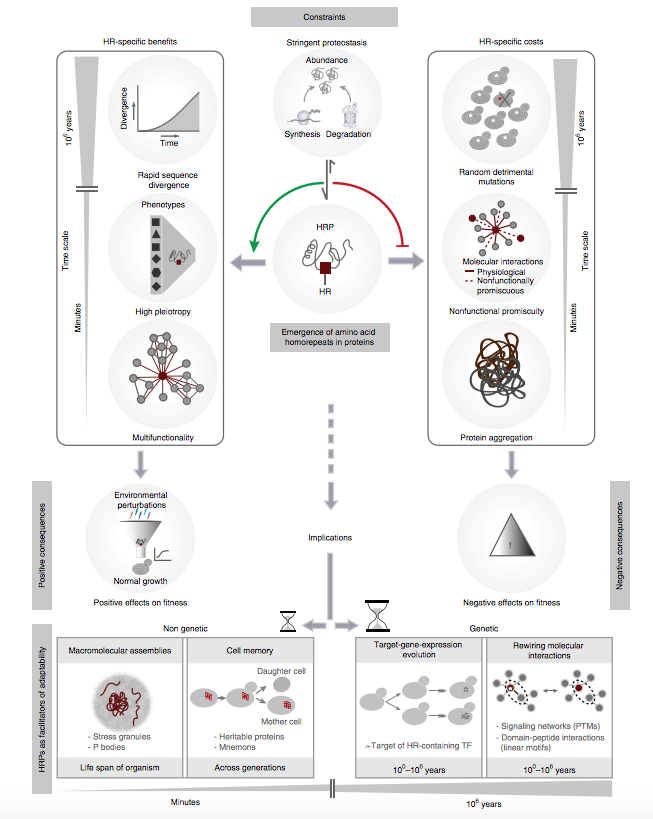
Proteins with amino acid homorepeats have the potential to be detrimental to cells and are often associated with human diseases. Why, then, are homorepeats prevalent in eukaryotic proteomes? In yeast, homorepeats are enriched in proteins that are essential and pleiotropic and that buffer environmental insults. The presence of homorepeats increases the functional versatility of proteins by mediating protein interactions and facilitating spatial organization in a repeat-dependent manner. During evolution, homorepeats are preferentially retained in proteins with stringent proteostasis, which might minimize repeat-associated detrimental effects such as unregulated phase separation and protein aggregation. Their presence facilitates rapid protein divergence through accumulation of amino acid substitutions, which often affect linear motifs and post-translational modification sites. These substitutions may result in rewiring protein interaction and signaling networks. Thus, homorepeats are distinct modules that are often retained in stringently regulated proteins. Their presence facilitates rapid exploration of the genotype– phenotype landscape of a population, thereby contributing to adaptation and fitness.
The paper by Chavali et al can be found here.