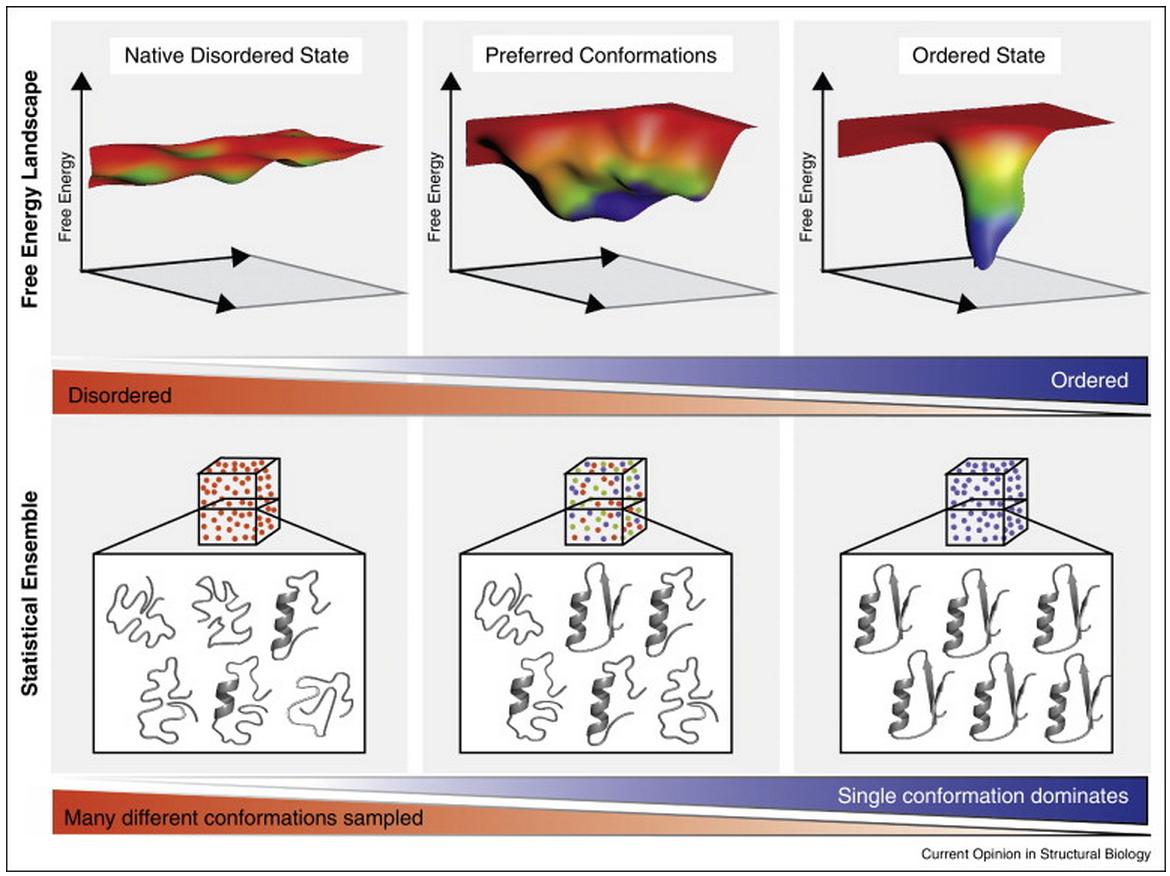
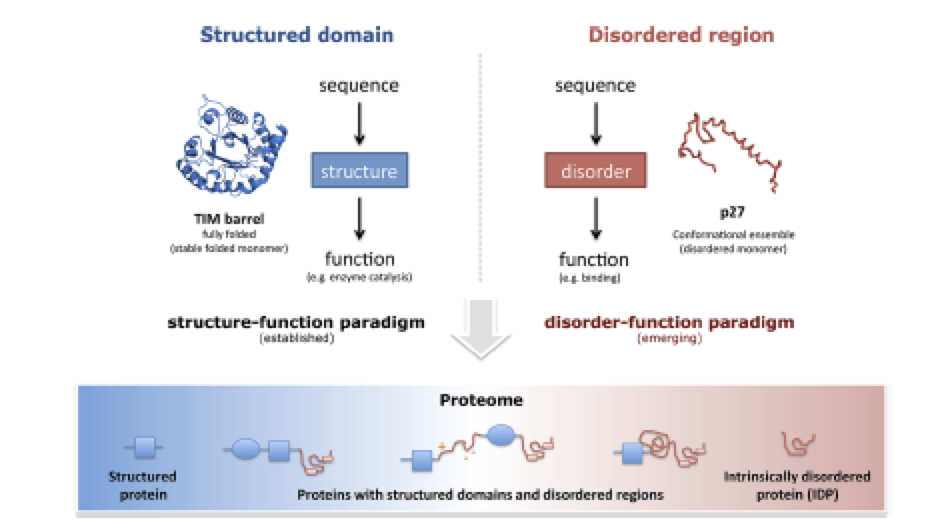
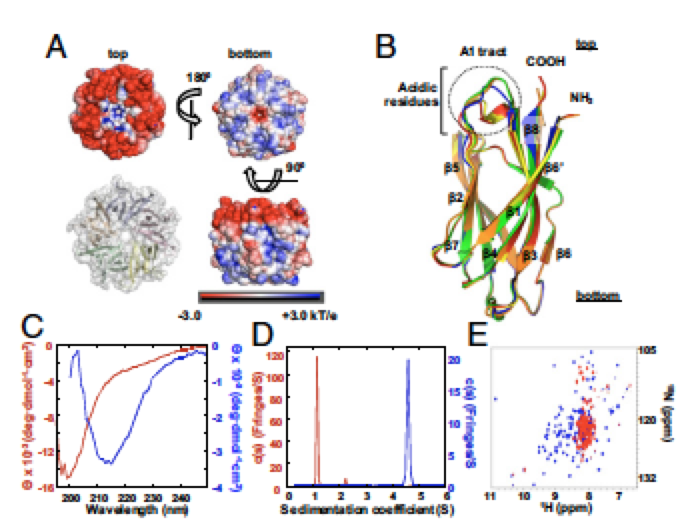
Controlling entropy to tune the functions of intrinsically disordered regions
Intrinsically disordered regions (IDRs) are fundamental units of protein function and regulation. Despite their inability to form a unique stable tertiary structure in isolation, many IDRs adopt a defined conformation upon binding and achieve their function through their interactions with other biomolecules. However, this requirement for IDR functionality seems to be at odds with the high entropic cost they must incur upon binding an interaction partner. How is this seeming paradox resolved? While increasing the enthalpy of binding is one approach to compensate for this entropic cost, growing evidence suggests that inherent features of IDRs, for instance repeating linear motifs, minimise the entropic cost of binding. Moreover, this control of entropic cost can be carefully modulated by a range of regulatory mechanisms, such as alternative splicing and post-translational modifications, which enable allosteric communication and rheostat-like tuning of IDR function. In that sense, the high entropic cost of IDR binding can be advantageous by providing tunability to protein function. In addition to biological regulatory mechanisms, modulation of entropy can also be controlled by environmental factors, such as changes in temperature, redox-potential and pH. These principles are extensively exploited by a number of organisms, including pathogens. They can also be utilised in bioengineering, synthetic biology and in pharmaceutical applications such as increasing bioavailability of protein therapeutics. The review by Tilman Flock, Robert J Weatheritt, Natasha S Latysheva and M Madan Babu can be found here.