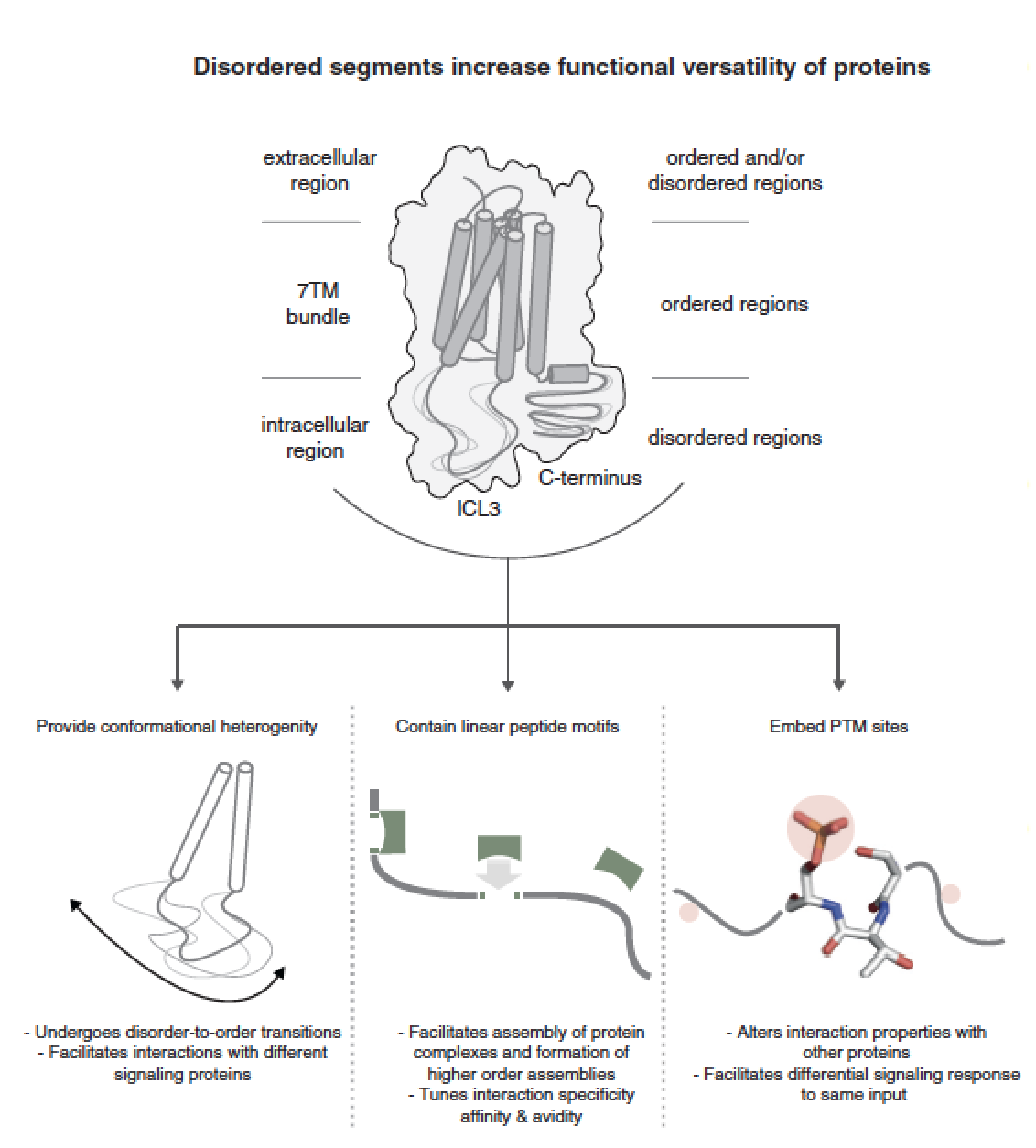
The seven-transmembrane (7TM) helix fold of G-protein coupled receptors (GPCRs) has been adapted for a wide variety of physiologically important signaling functions. Here, we discuss the diversity in the structured and disordered regions of GPCRs based on the recently published crystal structures and sequence analysis of all human GPCRs. A comparison of the structures of rhodopsin-like receptors (class A), secretin-like receptors (class B), metabotropic receptors (class C) and frizzled receptors (class F) shows that the relative arrangement of the transmembrane helices is conserved across all four GPCR classes although individual receptors can be activated by ligand binding at varying positions within and around the transmembrane helical bundle. A systematic analysis of GPCR sequences reveals the presence of disordered segments in the cytoplasmic side, abundant post-translational modification sites, evidence for alternative splicing and several putative linear peptide motifs that have the potential to mediate interactions with cytosolic proteins. While the structured regions permit the receptor to bind diverse ligands, the disordered regions appear to have an underappreciated role in modulating downstream signaling in response to the cellular state. An integrated paradigm combining the knowledge of structured and disordered regions is imperative for gaining a holistic understanding of the GPCR (un)structure-function relationship. The paper by AJ Venkatakrishnan et al can be viewed here.