Daniel and Alexey are our two new PhD students who started recently. A very warm welcome to both of them!


Daniel and Alexey are our two new PhD students who started recently. A very warm welcome to both of them!


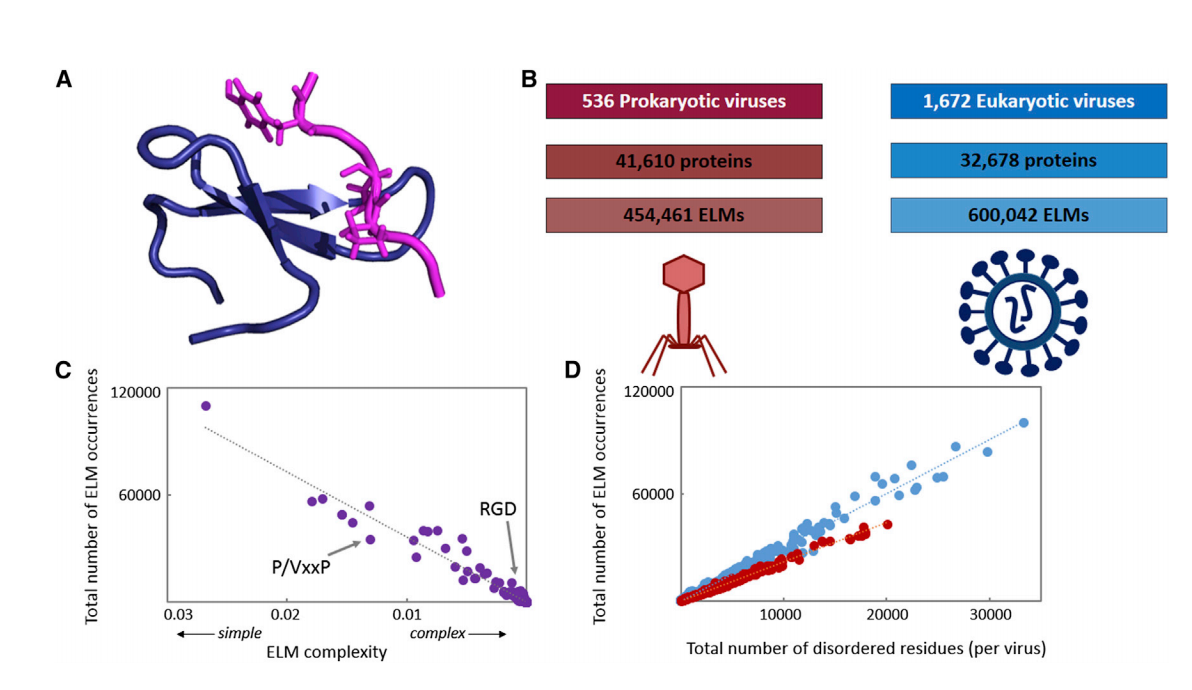
Viruses interact extensively with host proteins, but the mechanisms controlling these interactions are not well understood. We present a comprehensive analysis of eukaryotic linear motifs (ELMs) in 2,208 viral genomes and reveal that viruses exploit molecular mimicry of host-like ELMs to possibly assist in host-virus interactions. Using a statistical genomics approach, we identify a large number of potentially functional ELMs and observe that the occurrence of ELMs is often evolutionarily conserved but not uniform across virus families. Some viral proteins contain multiple types of ELMs, in striking similarity to complex regulatory modules in host proteins, suggesting that ELMs may act combinatorially to assist viral replication. Furthermore, a simple evolutionary model suggests that the inherent structural simplicity of ELMs often enables them to tolerate mutations and evolve quickly. Our findings suggest that ELMs may allow fast rewiring of host-virus interactions, which likely assists rapid viral evolution and adaptation to diverse environments. The paper can be found here.
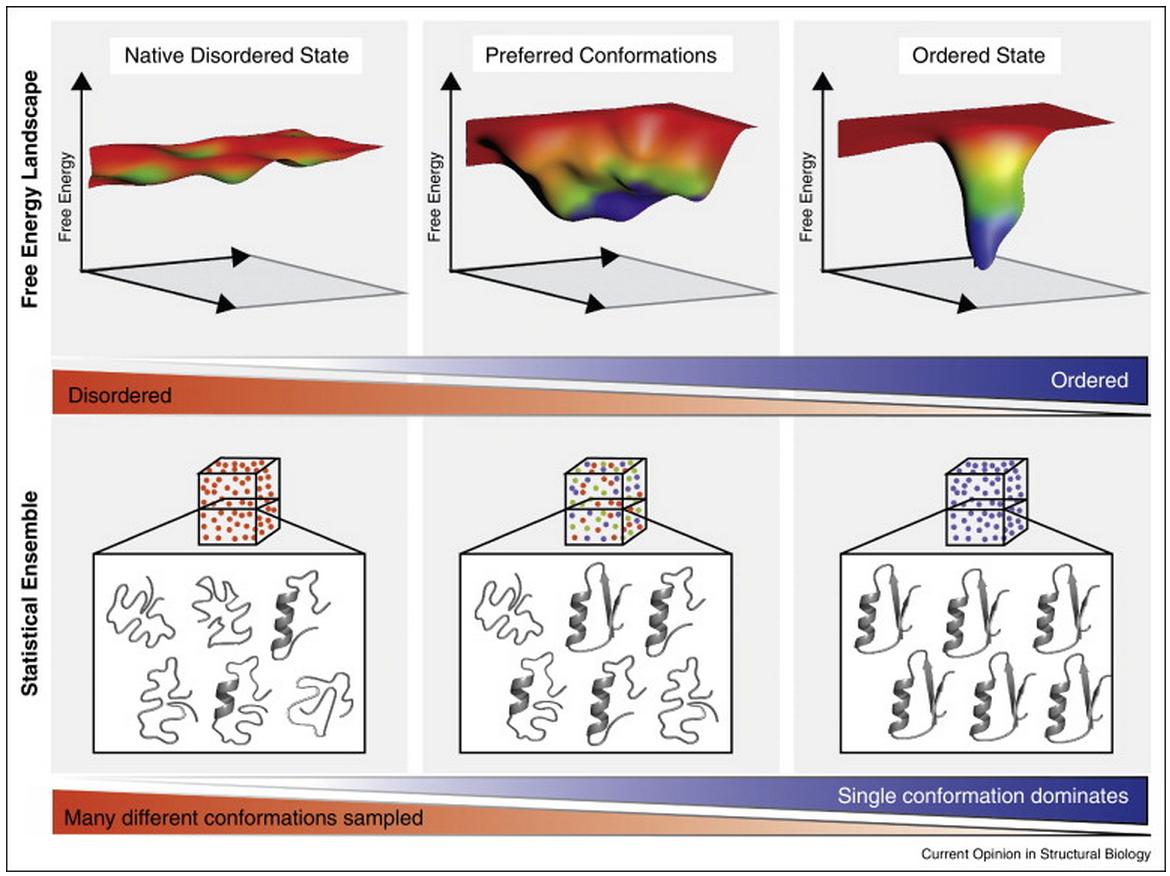
Intrinsically disordered regions (IDRs) are fundamental units of protein function and regulation. Despite their inability to form a unique stable tertiary structure in isolation, many IDRs adopt a defined conformation upon binding and achieve their function through their interactions with other biomolecules. However, this requirement for IDR functionality seems to be at odds with the high entropic cost they must incur upon binding an interaction partner. How is this seeming paradox resolved? While increasing the enthalpy of binding is one approach to compensate for this entropic cost, growing evidence suggests that inherent features of IDRs, for instance repeating linear motifs, minimise the entropic cost of binding. Moreover, this control of entropic cost can be carefully modulated by a range of regulatory mechanisms, such as alternative splicing and post-translational modifications, which enable allosteric communication and rheostat-like tuning of IDR function. In that sense, the high entropic cost of IDR binding can be advantageous by providing tunability to protein function. In addition to biological regulatory mechanisms, modulation of entropy can also be controlled by environmental factors, such as changes in temperature, redox-potential and pH. These principles are extensively exploited by a number of organisms, including pathogens. They can also be utilised in bioengineering, synthetic biology and in pharmaceutical applications such as increasing bioavailability of protein therapeutics. The review by Tilman Flock, Robert J Weatheritt, Natasha S Latysheva and M Madan Babu can be found here.
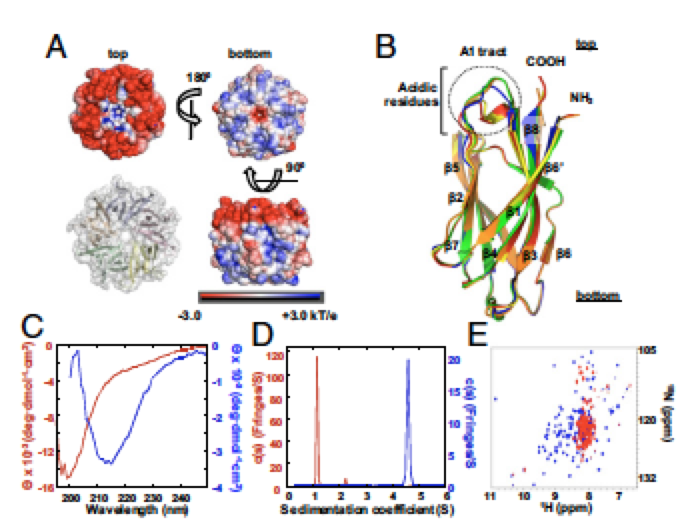
Nucleophosmin (NPM1) is a multifunctional phospho-protein with critical roles in ribosome biogenesis, tumor suppression, and nucleolar stress response. Here we show that the N-terminal oligomerization domain of NPM1 (Npm-N) exhibits structural polymorphism by populating conformational states ranging from a highly ordered, folded pentamer to a highly disordered monomer. The monomer–pentamer equilibrium is modulated by posttranslational modification and protein binding. Phosphorylation drives the equilibrium in favor of monomeric forms, and this effect can be reversed by Npm-N binding to its interaction partners. We have identified a short, arginine-rich linear motif in NPM1 binding partners that mediates Npm-N oligomerization. We propose that the diverse functional repertoire associated with NPM1 is controlled through a regulated unfolding mechanism signaled through posttranslational modifications and intermolecular interactions. The paper with Marija Buljan et al can be found here.
Channelling information flows: a young researcher’s approach to knowledge management
Guilhem is going to be a speaker at the PLS Conference in Paris which is held between 26-28 May 2014. His title “PLS Path Modelling of the molecular origins of gene expression noise” has been accepted for oral presentation.
Congratulations to Dr. M. Madan Babu for getting the Protein Science Young Investigator Award this year. The Protein Science Young Investigator Award, named for the academic journal of the Society, recognizes an important contribution to the study of proteins by a scientist still in the early stages of an independent career.

Ben has been awarded the EMBL Interdisciplinary Postdoc (EIPOD) fellowship to work at EMBL-Heidelberg. Many congratulations to Ben!
